Xiaohongshu Marketing Content Violation Policy 2023 - Part Two
Protecting Your Brand on Xiaohongshu: Compliance with Content Guidelines & Policy
It is important for brands and enterprises to be mindful of the types of violations that can occur when managing a Xiaohongshu account, particularly when it comes to content marketing. In the following sections, we will provide information on these violations and the platform’s content marketing rules. Our aim is to help users comprehend these rules, avoid misrepresentations, and enhance collaboration efficiency.
The sections covered will include:
- Common Types of Xiaohongshu Content Marketing Violations ⇐ Part One
- Xiaohongshu Content – Key Industry Policy
- Xiaohongshu Offensive Keywords Guideline
- Xiaohongshu Content – Frequently Asked Questions
- Xiaohongshu Marketing Appendix
Xiaohongshu Content - Key Industry Policy
Beauty and Personal Care Industry
It is not permissible to advertise product efficacy beyond the scope.
When promoting beauty and personal care products, it is important to consider whether the product belongs to special-use cosmetics. For special-use cosmetics, relevant product qualifications are required to promote its special efficacy.
Q: What products are considered special-use cosmetics?
A: Special-use cosmetics refer to cosmetics used for the purposes of whitening, hair growth, hair loss prevention, deodorization, etc. Common special-use cosmetics, such as whitening cosmetics, are considered special-use cosmetics, and brands need to provide relevant qualifications.
Q: How can one verify whether a product is a special-use cosmetic?
A: Check the “Cosmetics Query” section under the “Cosmetics” menu on the official website of the National Medical Products Administration for information on “Domestic Registration Information of Special-Use Cosmetics.”


Q: What product qualification proofs do merchants need to provide to the platform when promoting special-use cosmetics?
a) For domestic special-use cosmetics, provide the “Administrative License for Domestic Special-Use Cosmetics” and approval number;
b) For imported special-use cosmetics, provide the approval or registration certificate from the State Administration of Health, such as the “Administrative License for Imported Special-Use Cosmetics” or “Hygiene License and Approval Number for Imported Cosmetics.”
Q: How can merchants provide qualification proofs?
A: In the Pugongying backend, go to “My” -> “Qualification Management” -> “Product Category Qualifications.”
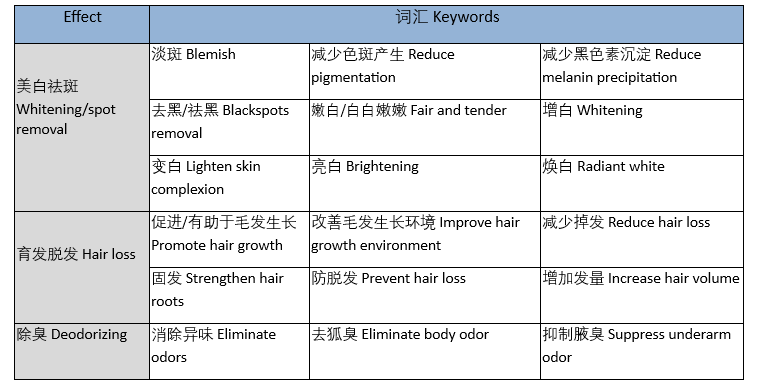
Q: What are some common keywords that belong to special efficacy descriptions of cosmetics?
A: Common efficacy-related keywords include but are not limited to the following, and relevant qualifications are required to mention them when promoting products.
Reminder Note:
Using medical terms in cosmetics and implying medical effects and efficacy is also considered advertising beyond the scope. Common examples include terms such as “medicinal,” “treatment,” “anti-allergy,” “antibacterial,” “fat-dissolving,” “slimming,” and various skin diseases or medical conditions.
Additionally, it is not permissible to make promises or guarantees about product or service efficacy, or engage in false or exaggerated advertising. When describing beauty and personal care products, it is important to avoid the common mistake of making promises to achieve a certain efficacy within a certain time frame, such as “whiten two degrees in ten days,” or making exaggerated claims about the product or service efficacy.

For example, the above note “Moisturizing and hydrating as fast as a tornado” makes an unrealistic guarantee about the effectiveness of the collaborative product, which is misleading to users.
Mistakes to avoid:
X – The king of whitening and milk-like skin
X – Finally can say goodbye to black and yellow faces
X – Wrinkles and fine lines, be gone, eraser is awesome!
X – The king of fading spots and whitening! After using it, your skin will really glow!
X – A few tens of dollars and my face is clean! Say goodbye to oily and acne-prone skin!

Have you all noticed the above issues?
Product efficacy descriptions should be truthful and reasonable, and avoid exaggeration or making promises. The above violations do not mean that these words are banned, but rather relate to their use in the current context, involving exaggerated descriptions.
Examples of compliant statements:
V – Even in an air-conditioned room, you can have dewy and beautiful skin
V – Whiter than envy-inducing, use this whitening mask
V – I’m convinced! This morning eye cream is the love of us night owls
V – Smelling fresh after showering! I thought I sprayed perfume
V – Oily and acne-prone skin raves about it!
Food Industry
Do not overstate the product efficacy in promotion.
Similar to the beauty and personal care industry, when cooperating with the food industry, attention should also be paid to whether the product belongs to health food. For health food, the brand can only mention the relevant health effects of the product in the cooperation notes after supplementing relevant qualifications on the platform.
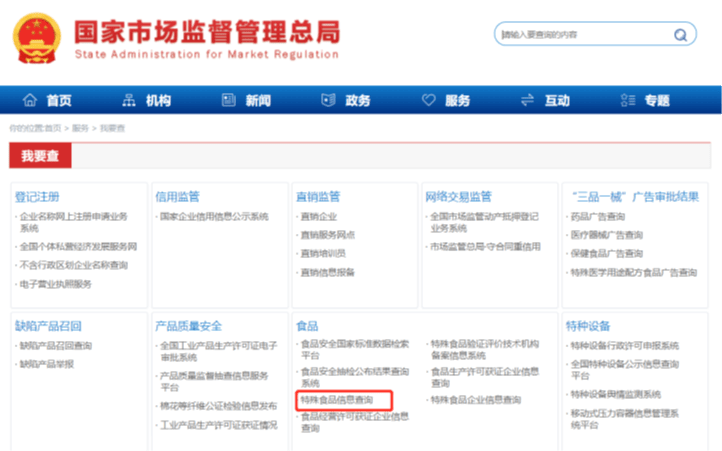
Q: How to verify if a product belongs to health food?
A: National Administration for Market Regulation – “Special Food Information Query” – “Registration of Health Food”

Q: What product qualification documents do businesses need to provide to the platform when promoting health food?
a) Main qualifications: The production enterprise provides the “Food Production License” (including health food); the business enterprise provides the “Food Business License” (including health food);
b) Product qualifications: Domestic production of health food provides the “Registration Certificate for Health Food” or the “Filing Certificate for Health Food”; imported health food provides the “Import Approval Certificate for Health Food”.
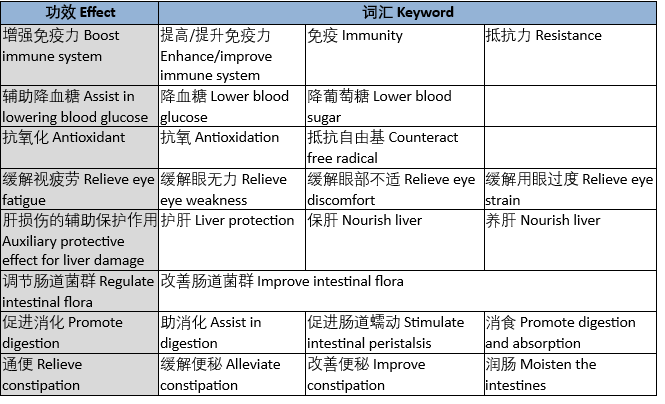
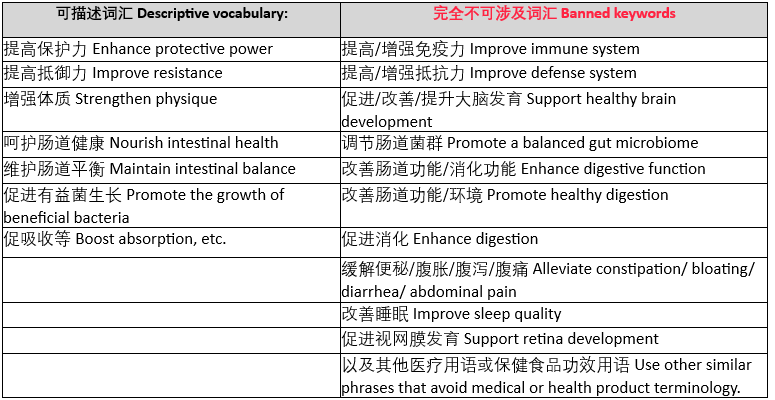
Q: Which common vocabulary belongs to the special efficacy description of health food?
A: Including but not limited to the following common efficacy-related vocabulary, cooperation products can only mention the relevant efficacy qualifications if they have them.
2. Attention needed for milk powder products:
a) It is prohibited to promote infant formula milk powder for babies under 3 years old;
b) Medical and healthcare efficacy claims are prohibited for infant formula and baby food;
c) It is prohibited to use children under 10 years old as advertising spokespersons for infant formula and baby food.
In addition, there are specific vocabulary review standards for milk powder products, including but not limited to the following:
Medical and Health Industry
In the medical and health industry, the following types of products or services require relevant qualifications to be provided to the platform before cooperating to promote:
Dental medical, medical beauty, medical beauty platform, physical examination institutions, postpartum care centers, online medical platforms, OTC drugs, medical equipment.
(Beauty contact lenses, massage equipment promoting pelvic repair, etc. all belong to medical equipment and require relevant product cooperation qualifications.)
Medical professionals, such as doctors/pharmacists/nurses, are prohibited from cooperating with three types of products (drugs, medical equipment, health food, special medical purpose formula food) and medical service industries (dental medical, medical beauty, postpartum care centers, etc.).
Common medical efficacy terms are as follows:
The following terms can be used for medical products with relevant qualifications:
• Anti-inflammatory, anti-allergy/desensitization, reduce redness
• Clear heat and remove dampness/moisture, promote blood circulation and nourish blood, promote metabolism
• Regulate endocrine, gastric distension and peristalsis, detoxify
Inflammation is a symptom of a disease and is a medical term. Variations such as “消火火” and “抗火火” also require relevant qualifications.
Next, we will share about Xiaohongshu Offensive Keywords Guideline. If you wish to know more about Xiaohongshu Marketing or China Marketing, please contact us with the form below!
Feel free to talk to us
It’s a team with one single shared goal, which is our client’s success. Deliver results for your business now.